Press Release
First Observation of Metamorphosis of an Enzyme that Catalyzes Two Chemical Reactions
November 11, 2011
Graduate School of Agricultural and Life Science, The University of Tokyo
High Energy Accelerator Research Organization (KEK)
Professor Takayoshi Wakagi and Associate Professor Shinya Fushinobu of the Graduate School of Agricultural and Life Sciences, the University of Tokyo and colleagues were the first to clarify how an enzyme of hyperthermophilic archaea origin that is thought to be positioned close to the origin of life can catalyze two reactions while metamorphosing itself.
Archaea possess many unusual enzymes. Among them, fructose-1,6-bisphosphate (FBP) aldolase/phosphatase is especially peculiar. In apparent violation of biochemical canons, this enzyme catalyzes two fundamentally different chemical reactions. Additionally, this enzyme is responsible for the biosynthesis of glucose, a process of critical importance in the early stage of the evolution of life.
The research team utilized the Photon Factory*1 at KEK to observe the enzyme’s structural metamorphosis to bring about the catalysis of the two different reactions. Such a multifunctional enzyme shatters long-held beliefs in biochemistry and raises the intriguing possibility that more such enzymes might be discovered in other organisms.
Background
Hyperthermophiles live in ultra-hot water and are positioned near the root of the evolutionary tree of life (Fig.1). As such, they are thought to be close to the common ancestor of life on Earth. Many hyperthermophiles are categorized as archaea, which belong to different branches from those of common organisms, such as bacteria and eukaryotes. Some hyperthermophilic archaea have the ability to build their own bodies by synthesizing complex compounds from simple inorganic substances, for example in the biosynthesis of saccharides from carbon dioxide. Reaction pathways in which organisms synthesize glucose are thought to be important in the evolution of primitive organisms.
For most common organisms two different enzymes, fructose-1,6-bisphosphate (FBP) aldolase and FBP phosphatase, are sequentially responsible for chemical reactions that lead to glucose production. By contrast, hyperthermophilic archaea make use of a single protein, termed FBP aldolase/phosphatase (FBPA/P), to catalyze these two reactions. This enzyme could be said to be bifunctional.
Methods and Resutls
The research team studied FBPA/P found in the hyperthermophilic archaeon, Sulfolobus, isolated from hot water at Beppu Onsen in Oita Prefecture, Japan. The three-dimensional structure of FBPA/P looks similar to a barrel of eight identical molecular units. Reactions are catalyzed in the regions between these units (Fig. 2).
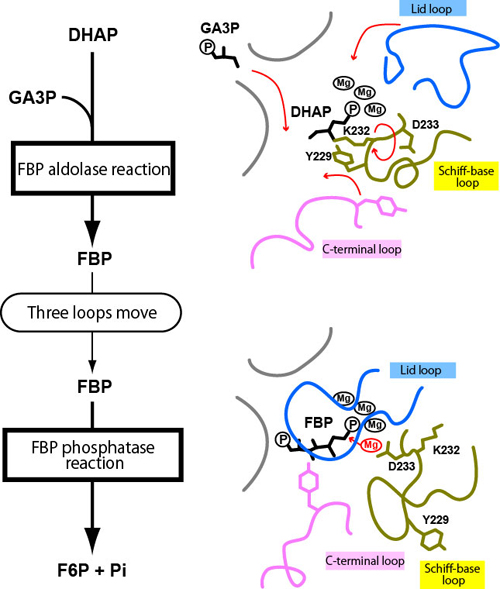
The two reactions that FBPA/P catalyzes are: 1) FBP aldolase reaction, in which dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (GA3P) produce FBP and 2) FBP phosphatase reaction, in which FBP is cleaved into fructose-6-phosphate (F6P) and inorganic phosphate (Pi) (Fig. 3 left).
In 2004, the research team elucidated the three-dimensional structure of the enzyme-substrate complex while FBPA/P is catalyzing the FBP phosphatase reaction (Fig. 3 bottom right; FBP is bound). In the present study, the same group succeeded in unraveling the three-dimensional form of the enzyme-substrate complex while FBPA/P is catalyzing the FBP aldolase reaction (Fig. 3 top right; DHAP is bound) by using X-ray crystallography*2 in their experiments performed at the AR-NW12A beamline of the KEK’s Photon Factory. Comparison of these two forms revealed that the active site (the region of an enzyme in which a chemical reaction is catalyzed) of FBPA/P metamorphoses into two completely different forms owing to the extensive movements of three loops (lid loop, Schiff-base loop, and C-terminal loop).
In the FBP aldolase form, a lysine residue (K232) binds to the first substrate, DHAP. When the second substrate, GA3P, enters the active center, a neighboring tyrosine residue (Y229) is thought to mediate reaction of GA3P and DHAP into FBP (FBP aldolase reaction). Once FBP is produced, the lysine residue becomes free and the three loops can move. The Schiff-base loop is flipped over and both the lid and C-terminal loops close, metamorphosing the enzyme into its FBP phosphatase form. Next, an aspartate residue (D233) enters the active center, and binds magnesium ions, thereby triggering the dissociation of FBP into F6P and Pi (FBP phosphatase reaction).
This finding revealed that such a ‘metamorphosis’ is the key to the catalysis of two different reactions for a given enzymatic reaction sequence. This mechanism turns on its head the established biochemical dogma that describes one enzyme as catalyzing only one reaction.
Common organisms of more recent ancestry do not possess FBPA/P, but rather make use of two different enzymes separately to catalyze the two reactions independently. It is possible that primitive organisms might perform biosynthesis in a simpler manner than more contemporary organisms do by utilizing bifunctional enzymes such as FBPA/P.
Future Prospect
The present study is the first to reveal the mechanism by which one enzyme may catalyze two different chemical reactions. Moreover, the current finding hints at the possibility that more multifunctional enzymes similar to FBPA/P may exist. In addition, it can be expected that more enzymes capable of converting simple starting materials into synthetically useful intermediates will be discovered.
Publication
Shinya Fushinobu*, Hiroshi Nishimasu*, Daiki Hattori, Hyun-Jin Song, and Takayoshi Wakagi†, “Structural basis for the bifunctionality of fructose 1,6-bisphosphate aldolase/phosphatase.” Nature, Online Edition. October 10, 2011. DOI:10.1038/nature10457.
*First author these authors contributed equally, †Primary investigator
Figure 1. Outline of the evolutionary tree of life. Hyperthermophiles (indicated in red) are near the bottom parts of archaeal and bacterial branches and make use of FBPA/P.
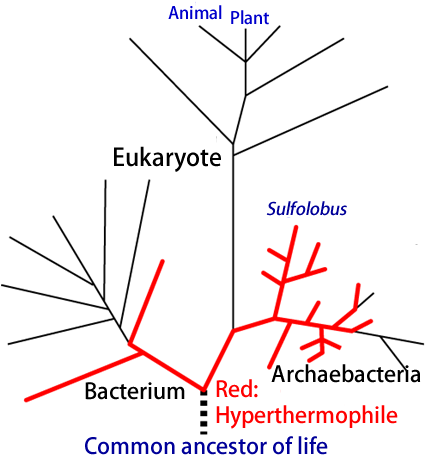
Figure 2. Total three-dimensional structure of FBPA/P. Eight identical units gather to form a barrel-like structure. Each unit is represented using an iridescent color (blue, light blue, green, yellowish green, orange, and red). Bound DHAP bases and magnesium ions are depicted as purple and pink spheres, respectively.
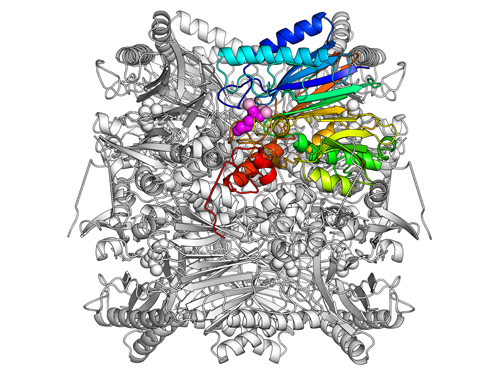
Figure 3. Reactions that are catalyzed by FBPA/P (left) and schematic views of regions where reactions occur (right). Three loops undergo significant conformational changes in changing between the FBP aldolase form (top right) and the FBP phosphatase form (bottom right).
Glossary
*1 Photon Factory (PF)
Photon Factory (PF) at KEK, Tsukuba is the first synchrotron light source for the X-ray region in Japan. Since its commission in 1982, PF has been upgraded several times to provide high quality X-rays. Equipped with cutting-edge experimental apparatus, PF has been providing numerous advanced scientific findings in materials and life science.
*2 X-ray crystallography
One of the most common techniques to determine the three-dimensional structure of enzymes (proteins). Sample crystals are irradiated with X-rays and diffraction data are measured to determine the precise three-dimensional structure of the sample.
[ Media Contact ]
Graduate School of Agricultural and Life Science, The University of Tokyo
Prof. Takayoshi Wakagi
Tel:+81-03-5841-5152
E-mail:atwakag@mail.ecc.u-tokyo.ac.jp
URL:http://enzyme13.bt.a.u-tokyo.ac.jp/index-e.html
Graduate School of Agricultural and Life Science, The University of Tokyo
Associate Prof. Shinya Fushinobu
Tel:+81-03-5841-5151, +81-03-5841-8227
E-mail:asfushi@mail.ecc.u-tokyo.ac.jp
Hitoshi Abe
Graduate School of Agricultural and Life Science, The University of Tokyo PRoffice
Tel: +81-03-5841-8179
E-mail: koho@ofc.a.u-tokyo.ac.jp
Youhei Morita
Public Relations Office
High Energy Accelerator Research Organization (KEK)
Tel: +81-29-879-6047
E-mail: press@kek.jp