Press Release
The First Elucidation of the Mechanism of Fault Slips That Do Not Cause Large Earthquakes
2 Nov, 2011
Tokyo Institute of Technology, Japan
Ochanomizu University, Japan
High Energy Accelerator Research Organization, Japan
Okayama University, Japan
Assistant Professor Hiroshi Sakuma of Tokyo Institute of Technology, Professor Toshihiro Kondo of Ochanomizu University, Associate Professor Hironori Nakao of the High Energy Accelerator Research Organization (KEK), Professor Katsuyuki Kawamura of Tokyo Institute of Technology (currently at Okayama University), and a colleague are the first to elucidate one of the mechanism by which mineral surfaces are lubricated due to adsorbed water. This process provides insight into the phenomenon of creeping faults,*1 which continuously slip without causing large earthquakes. This elucidation was achieved using the Photon Factory (PF) at KEK, Japan.
This research team performed experiments with muscovite, which is ubiquitous in igneous rocks and has a structure similar to clay minerals contained in faults. The team immersed the surface of muscovite in a sodium chloride solution and observed that sodium ions are adsorbed on its surface. Moreover, the team clarified that these adsorbed sodium ions are surrounded by water molecules as the inner sphere complexes and the ions maintain the layers of water molecules aroud them, thereby exhibiting a high lubricating property.
A systematic understanding of the effects of water on the lubricating property between minerals in faults is needed to establish the fundamental physics of all faults, including creeping faults.
Results of the present study were published in the Journal of Physical Chemistry C.
Background
Fracture of rocks and fault slip are triggered by the movement of tectonic plates and cause earthquakes. However, there is a fault termed “creeping fault ” that continuously slips extremely slowly without causing large earthquakes. One possible reason of the continuous slip has been proposed as the low friction between layered clay minerals and lubrication adsorbed water molecules onto the mineral surfaces. Dr. Sakuma and colleagues have experimentally demonstrated that sodium chloride (NaCl) solution can be a good lubricant when the solution is confined between muscovite (mica) surfaces even when it is compressed down to a thickness of 1 nm or less. Muscovite is ubiquitous in igneous rocks and the structure is similar to clay minerals often found in faults. The 1 nm corresponds roughly to the three times diameter of a water molecule. However, the mechanism of this lubrication had not been understood. This research team tried to understand the electronic states of the muscovite/NaCl solution interface because the information should be strongly related to the mechanism of the lubrication.
Methods and Resutls
This research team investigated the electron density of the interface between muscovite and sodium chloride solution using an X-ray crystal truncation rod (CTR) scattering technique*2 and converted the electron density to the densities of chemical elements by molecular dynamics (MD) simulations*3. The X-ray CTR scattering technique can reveal the electron density profiles of solid/liquid interfaces with a resolution of 0.1 nm or less. However, this technique alone cannot directly provide the information of atomic species. Therefore, the distribution of the chemical elements at the interface was obtained by comparing the results of MD calculations using a precise interatomic potential model with those of the X-ray CTR scattering. X-ray CTR scattering experiments were carried out at the BL-4C X-ray diffraction experiment station for material science of Photon Factory (PF) in KEK, and MD simulations were performed using the interatomic potential model that was originally developed by Drs. Sakuma and Kawamura and their colleagues. These experiments and simulations revealed the pictures of the muscovite/NaCl solution interface as follows:
(1) The oscillation of the electron density profile of sodium chloride solution is observed within 1.2 nm from the muscovite surface (Fig. 1).
(2) Hydrated sodium ions adsorbed on the negatively charged muscovite surface as the inner sphere complexes (Fig. 2).
(3) The radii of the first hydration shell*4 of the adsorbed hydrated sodium ions extend to ~0.5 nm from the muscovite surface.
These results provide a plausible mechanism for the high lubrication between muscovite surfaces due to the existence of sodium chloride solution. When the sodium chloride solution is compressed down to a thickness of 1 nm or less between two opposite muscovite surfaces, the first hydration shells of the hydrated sodium ions adsorbed onto the muscovite surface on one side makes contact with that on the opposite side. The hydrated sodium ions surrounded by water molecules act like a ball-bearing between muscovite surfaces and provide the low friction between the surfaces. Results of the present study were published in the Journal of Physical Chemistry C, an academic journal of the American Chemical Society. The title is “Structure of Hydrated Sodium Ions and Water Molecules Adsorbed on the Mica/Water Interface”
Future Prospect
A systematic understanding of the effects of water on the lubricating property of various minerals in faults is important to establish the fundamental physics of faults, including creeping faults. A mechanism proposed here to explain the low friction between muscovite surfaces due to the adsorbed water molecules provides new perspective to studies in this field and will contribute to the development of future studies. One important future subject is to know the effects of high temperature and differential stress. The group has found that hydrated sodium ions confined between muscovite surfaces can stably exist even under the differential stress of dozens MPa (1 MPa = 10 atm). To know the effects of temperature and stress over the wide ranges of the earth’s crust will lead to the advances in the research fields of faults and earthquakes.
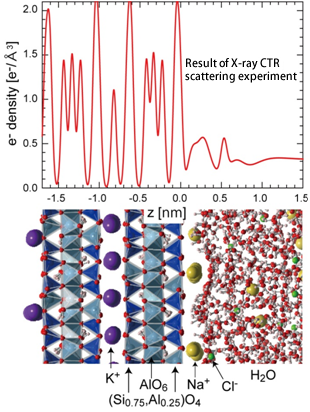
Figure 1. Electron density profile of muscovite and sodium chloride solution (0.5 mol/L) interface. The bottom figure shows a snapshot of the atomic distribution obtained from MD simulations and the atomic species corresponding to electron density profiles can be deduced from the comparison between top and bottom figures.
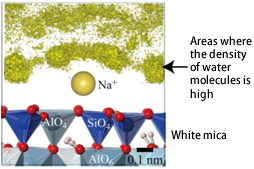
Figure 2. A hydrated sodium ion adsorbed onto a muscovitemuscovite surface. Yellow isodensity surfaces indicate the high water density.
Contact Information
About the details of the present studyAssistant Professor Hiroshi Sakuma
Department of Earth and Planetary Sciences, Graduate School of Science and Engineering, Tokyo Institute of Technology
TEL: 81-3-5734-3636
FAX: 81-3-5734-3636
E-mail: sakuma.h.aa@m.titech.ac.jp
Professor Toshihiro Kondo
Division of Advanced Sciences, Graduate School of Humanities and Sciences, Ochanomizu University
TEL: 81-3-5978-5347
E-mail: kondo.toshihiro@ocha.ac.jp
Associate Professor Hironori Nakao
Institute of Materials Structure Science, High Energy Accelerator Research Organization
TEL: 81-29-879-6025
E-mail: hironori.nakao@kek.jp
Professor Katsuyuki Kawamura
Division of Sustainability of Resources, Graduate School of Environmental Science, Okayama University
TEL: 81-86-251-8845
E-mail: kats@cc.okayama-u.ac.jp
Public Relations
Evaluation and Public Relations Division
Tokyo Institute of Technology
TEL: 81-3-5734-2975
E-mail: hyo.koh.sya@jim.titech.ac.jp
Public Relations Office
High Energy Accelerator Research Organization
TEL: 81-29-879-6047
E-mail: press@kek.jp
Public Relations Section, Public Relations Office, Academic and Information Board
Ochanomizu University
TEL: 81-3-5978-5104
E-mail: info@cc.ocha.ac.jp
Public Relations Office, Planning and Public Information Division
Okayama University
TEL: 81-86-251-7292
E-mail: www-adm@adm.okayama-u.ac.jp
Glossary
*1 Creeping faults
Faults in which an aseismic fault slip occur. Creeping faults as part of the San Andreas Fault are famous, and the continuous slip velocities are approximately 5 mm/year.
*2 X-ray crystal truncation rod (CTR) scattering technique
One of surface X-ray diffraction techniques. When X-rays are irradiated onto a crystal surface, the X-rays are scattered by electrons in atoms, producing a weak regular scattering pattern between Bragg reflection points. By analyzing this pattern, the atomic arrangement in the surface can be revealed.
*3 Molecular dynamics (MD) calculation
A numerical calculation technique in statistical mechanics. Forces between atoms, molecules, or both are calculated from the interaction energy between them. By solving equations of motion for these atoms and molecules, stable structures and physical properties of the material can be predicted.
*4 First hydration shell
An area of the high density of the closest water molecules surrounding an ion. The physical properties of these water molecules can be different from the other water molecules due to thestrong electrostatic interaction with the ion.