Press Release
To what extent can aerosols cool the Earth?
Aug 23, 2011
Hiroshima University, Japan
High Energy Accelerator Research Organization (KEK), Japan
Professor Yoshio Takahashi, graduate student Takema Furukawa and their colleagues at the Department of Earth and Planetary Systems Science, Graduate School of Science, Hiroshima University, Japan, have found that the cloud-forming activity of organic aerosols*1 is less than previously predicted. Organic aerosols are known to absorb moisture and thereby help form clouds. Using the Photon Factory at the High Energy Accelerator Research Organization (KEK; Tsukuba, Japan) and SPring-8 (Nishi-Harima, Japan), Professor Takahashi and his colleagues have now made a more precise estimate of this cloud-forming activity.
This finding is expected to improve the accuracy of calculating the global cooling effects of aerosols and thus make a significant contribution to the quantitative prediction of global warming.
1. Background
Global warming is perhaps the most consequential environmental issue facing the world today. Accurate prediction of global climate change is the key to promoting efforts to stem global warming. The Intergovernmental Panel on Climate Change (IPCC; Nobel Peace Prize winner in 2007) reported that cloud formation by aerosols, small particles suspended in the atmosphere, has global cooling effects.
Aerosols reflect and block sunlight when they are suspended in the atmosphere, and thus cool the Earth (Direct cooling effects, Fig. 1). Additionally, aerosols containing highly hygroscopic particles promote cloud formation by serving as nuclei for cloud condensation. The resulting clouds also reflect sunlight and thus cool the Earth, suggesting that highly hygroscopic aerosols also contribute indirectly to preventing global warming (Indirect cooling effects, Fig. 1). The IPCC has examined these two types of cooling effects separately to quantitatively evaluate their ability to mitigate global warming. The IPCC claims that the indirect cooling effects of cloud formation are more significant than the direct effects; however, large errors due to uncertainty about the composition and hygroscopicity of aerosols lead to large uncertainties in estimates of the global cooling effects of aerosols (Fig. 2). Thus, a more precise calculation of aerosol hygroscopicity and the resulting cloud-forming activity is a key requirement for improving the scientific reliability of global warming effects of aerosols.
2. Research Methods and Results
Particles that contribute to the indirect effects are divided into two categories, inorganic aerosols (such as sulfates) and organic aerosols. Oxalic acid is a major component of organic aerosols. Using X-ray absorption fine structure (XAFS) spectroscopy*2 at the Photon Factory at KEK and SPring-8, Professor Takahashi and colleagues have found that the majority of oxalic acid in aerosols forms metal complexes*3 with ions such as calcium and zinc (Fig. 3). This discovery is important for the following reasons:
- 1. The hygroscopicity of oxalic acid decreases by more than 99% when it forms a complex with calcium or zinc.
- 2. When the hygroscopicity decreases, the cloud-forming activity of oxalic acid decreases.
- 3. Therefore, the fact that oxalic acid exists as a metal complex indicates that indirect cooling effects of oxalic acid are not actually as large as assumed in previous predictions.
The result of the present study was published in the online edition of Atmospheric Chemistry and Physics, an academic journal published by the European Geosciences Union, in May 2011.
Publication
"Oxalate metal complexes in aerosol particles: implications for the hygroscopicity of oxalate-containing particles", Atmospheric Chemistry and Physics (online edition, May 10, 2011).
3. Future Prospects
The result of the present study suggests that other dicarboxylic acids and organic acids may well form metal complexes, indicating that complex formation with metal ions should be considered when estimating the cooling effects of water-soluble organic aerosols.
This finding is expected to contribute to the more precise prediction of global warming as well as precise quantification of the global cooling effects of aerosols, which will be conducted by the IPCC and other organizations.
[ Media Contact ]
Yoshio Takahashi:
Hiroshima University
Tel: 81-82-424-7460 E-mail: ytakaha@hiroshima-u.ac.jp
Tomoyo Okada:
Public Relations Office,
Hiroshima University
Tel: 81-82-424-6017 E-mail: koho@office.hiroshima-u.ac.jp
Youhei Morita:
Public Relations Office,
High Energy Accelerator Research Organization, Japan
Tel: 81-29-879-6047 E-mail: press@kek.jp
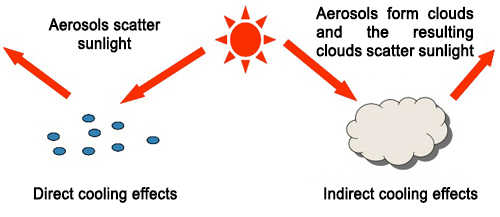
Figure 1. Global cooling effects of aerosols.
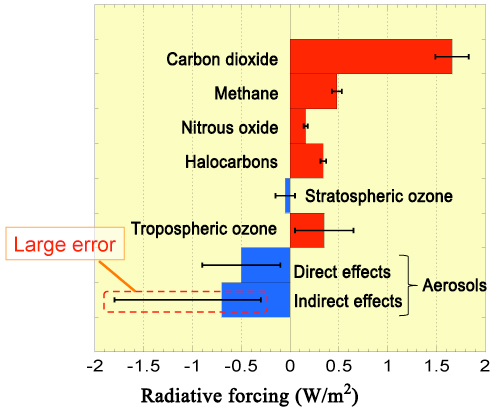
Figure 2. Contributions of several chemical compounds to global warming between 1975 and 2005.
Reproduced from the data reported in the 4th assessment report of the IPCC (2007). Radiative forcing: red bars indicate contributions to warming, while blue bars indicate contributions to cooling.
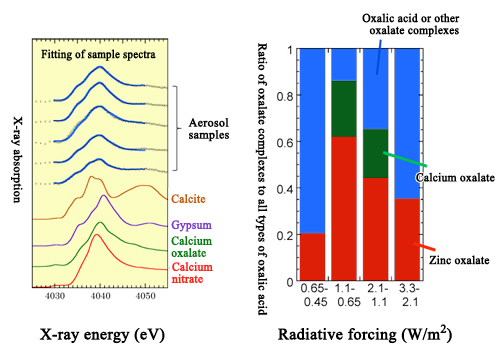
Figure 3. Identification of calcium oxalate by K-edge XAFS (left) and the ratios of calcium complexes and zinc complexes to all types of oxalic acid (right: plotted against diameter of aerosols).
All aerosol samples were collected in Tsukuba, Japan.
Glossary
- *1 Organic aerosols
- Special types of aerosols (small particles suspended in the atmosphere, such as dust and cigarette smoke) comprised of organic compounds.
- *2 X-ray absorption fine structure (XAFS) spectroscopy
- Experimental technique to measure absorption spectra of materials by varying the X-ray energy. Chemical states and structures of samples can be determined by analyzing measured spectra.
- *3 Metal complexes
- Compounds in which non-metal and metal atoms are bonded. The specific metal complexes discussed in this document involve oxalic acid (a non-metallic organic compound) and calcium or zinc (metal).