Press Release
Successful Development of the World’s Fastest Soft X-ray Absorption Spectroscopic Technique that Can Identify the Chemical Species and Quantities of Molecules Adsorbed on the Surface
—A Powerful Technique to Elucidate the Mechanism of Catalysis—
January 27, 2012
High Energy Accelerator Research Organization(KEK), Japan
Keio University, Japan
Associate Professor Kenta Amemiya of the Institute of Materials Structure Science at the High Energy Accelerator Research Organization (KEK) and colleagues have developed in collaboration with a group led by Professor Hiroshi Kondo of Keio University School of Science and Technology, the world’s fastest soft X-ray absorption spectroscopy to continuously measure the chemical species and quantities of molecules contained in one or less monolayer*1 unit of the surface of a solid. This novel technology allowed the research team to observe the fast chemical reactions that occur on the surface of catalysts, such as those used in filtering exhaust gases from automobiles. Such an understanding enables the elucidation of the mechanisms of catalytic reactions and facilitates the improvement of the next generation of high performance catalysts.
|
1. Background
The exhaust emitted by automobile engines contains toxic gases including carbon monoxide (CO) and nitrogen oxides (NOx). Catalysts are materials that may convert these toxic gases into non-toxic forms. Catalytic converters clean exhaust gases nearly instantaneously as the exhaust gases pass over them. Catalysts utilized in such catalytic converters mounted on automobiles are solid and are referred to as heterogeneous catalysts*2. Toxic gas molecules, which react on the surface of these solid catalysts, are converted into non-toxic molecules and exit the catalytic converters.
To study the details of reactions mediated by heterogeneous catalysts can provide important clues towards the development of high performance catalytic converters. To this end, spectroscopic*3 techniques that can perform direct measurements of the types and quantities of molecules on the outermost surface of solids are required. Soft X-ray absorption spectroscopy is one such technique, capable of identifying the chemical species of molecules at a scale as small as 1/100 of one monolayer unit. However, as it can take several minutes to obtain a single datum using soft X-ray absorption, the study of rapid reactions that occur on catalyst surfaces is precluded. To overcome this difficulty, the research team developed a novel technique—wavelength-dispersive soft X-ray absorption spectroscopy—to study the rapid chemical reactions that occur on catalyst surfaces at speeds of 30 frames per second, the same speed through which images are processed in motion pictures.
2. Methods and Results
In the wavelength-dispersive soft X-ray absorption spectroscopy, soft X-rays with different wavelengths are irradiated upon a sample depending on sample’s surface positions, i.e., wavelength-dispersive soft X-rays illuminate the sample. Electrons emitted via the absorption of the irradiated X-rays at each position are separately detected (Fig. 1), allowing researchers to detect the absorption of various wavelengths of X-rays at a time. The chemical species and quantities of molecules on sample’s surface can be precisely determined from spectra that show the wavelength (energy) dependent absorption patterns of irradiated soft X-rays.
In the present study, this spectroscopic technique has been utilized along with highly brilliant soft X-rays obtained from an undulator*4 placed on the BL-16A beamline at the KEK’s Photon Factory, allowing the research team to measure real-time chemical reactions at high speed (30 spectra per second). Consequently, continuous observation of all the details of fast chemical reactions such as those occurring on the surface of catalysts has become possible.
The research team studied the real-time chemical reactions of carbon monoxide (CO) and oxygen (O) that occurred on the surface of iridium (Ir), amongst the most fundamental of catalytic reactions (Fig. 2). In this study, atomic O with a thickness of one or less monolayer unit was adsorbed onto an Ir sample, and then catalytic reactions were induced by flowing CO over it. Upon the initiation of CO flow, an O peak drastically diminished and a CO peak grew at the same time on the absorption spectra. This is due to the consumption of O in a chemical reaction, CO + O → CO2. Atomic O was exhausted in 150 seconds after the initiation of the reaction, and only CO remained on the Ir surface. By analyzing this data, temporal changes of the quantities of CO and O on the Ir surface under reaction can be precisely evaluated (Fig. 3(a)). Moreover, all reactions were completed within 10 seconds at 277℃ (Fig. 3(b)), which is close to the temperature above which catalytic converters on automobiles start functioning effectively. Thanks to high-speed recording at 30 frames per second, details about temporal changes of the quantities of CO and O could be observed throughout.
As described above, the development of the wavelength-dispersive soft X-ray absorption spectroscopy has made it possible to measure not only catalytic reactions but also various other chemical reactions that occur on a solid surface at a speed similar to that with which images are processed in motion pictures. This allows the precise determination of the types and quantities of molecules involved in these reactions.
The present study was conducted under Program G of the Institute of Materials Structure Science of KEK (No. 2010G146) as part of the Quantum Beam Technology Program of the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan. The research team also received the financial support of the Grant for Private Education Institute Aid from MEXT.
Publication
K. Amemiya, Y. Kousa, S. Nakamoto, T. Harada, S. Kozai, M. Yoshida, H. Abe, R. Sumii, M. Sakamaki, and H. Kondoh, “Real-time observation of CO oxidation reaction on Ir(111) surface at 33 ms resolution by means of wavelength-dispersive near-edge x-ray absorption fine structure spectroscopy.” Applied Physics Letters. August 15, 2011. [doi:10.1063/1.3624587]
3. Future Prospects
It is expected that applications of this technique will lead to the development of novel catalysts with higher performance via the elucidation of the mechanisms of various catalytic reactions. Moreover, at the BL-16A beamline, where the present study was conducted, a polarization switching*5 technique is being developed. In combination, these two techniques could not only identify the chemical species and quantities of molecules involved in a reaction, but also whether and how the orientation of molecules changes over the course of the reaction. These collective insights will all be necessary to both illuminate current paradigms of catalytic reaction mechanisms as well as position us to make breakthroughs in the next generation of catalysts.
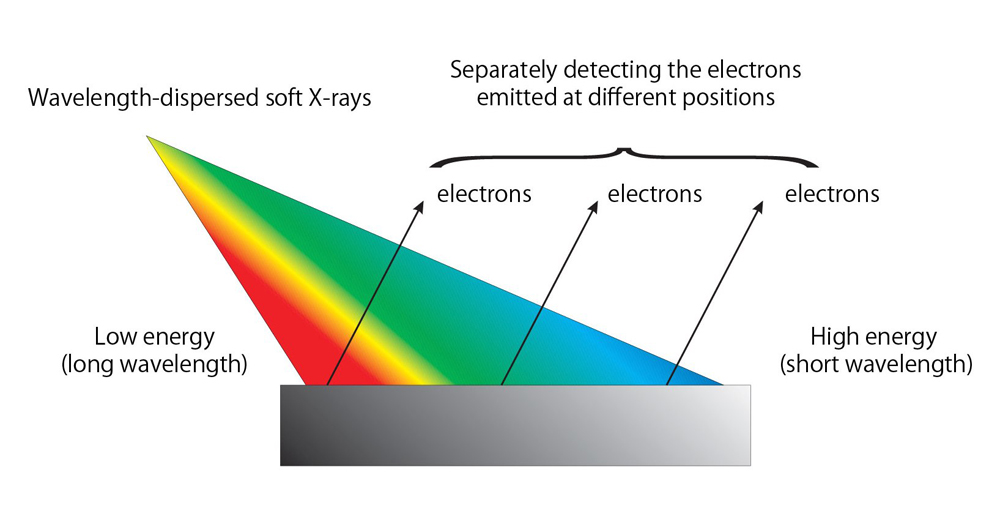
Figure 1. Schematic view of wavelength-dispersive soft X-ray absorption spectroscopy
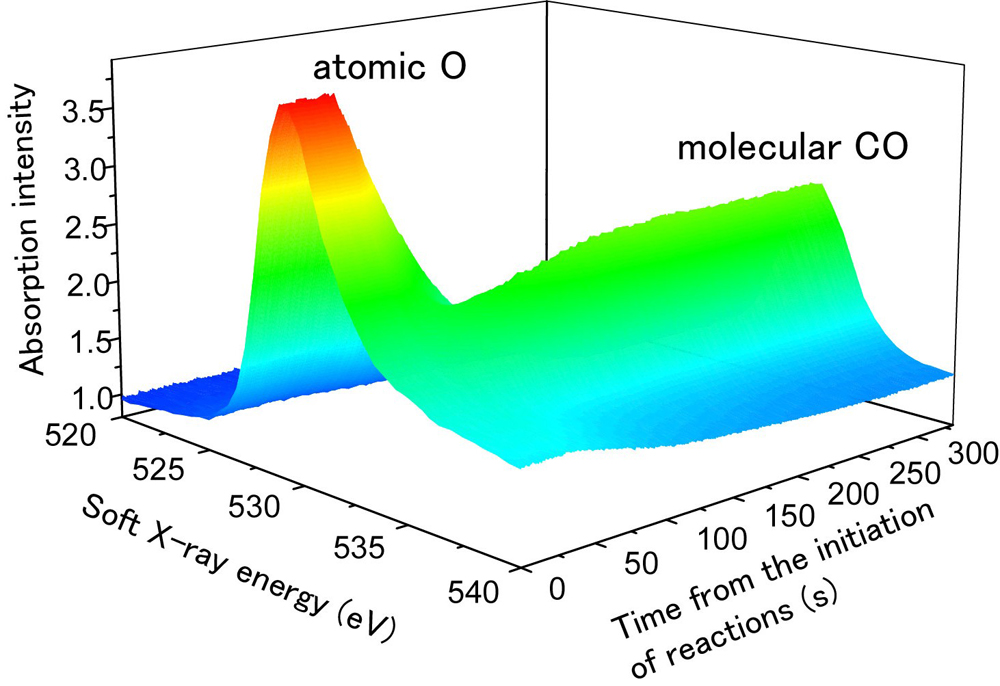
Figure 2. Temporal changes of soft X-ray spectra measured as oxygen (O) and carbon monoxide (CO) react on an iridium (Ir) surface.
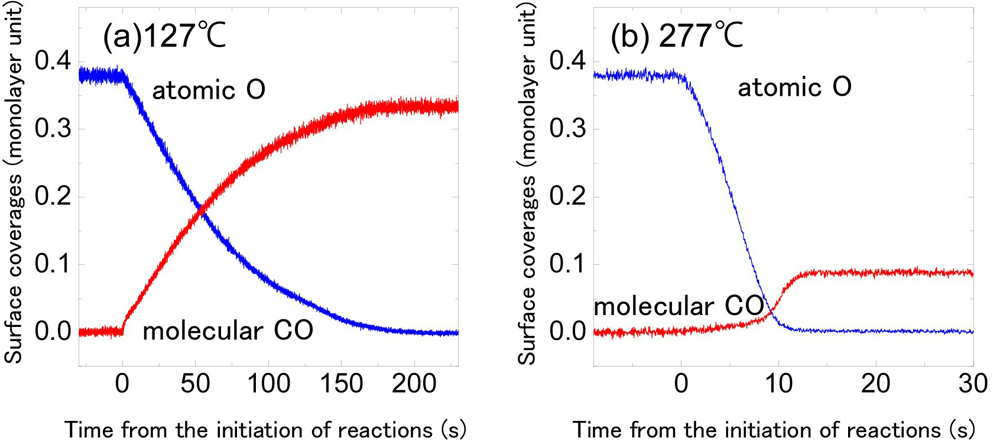
Figure 3. Temperature dependence of the evolution over time of O and CO coverages on an iridium (Ir) surface.
Media Contact
[for research]
Associate Professor Kenta Amemiya
Institute of Materials Structure Science,
High Energy Accelerator Research Organization, Japan
Tel: 81-29-864-5656
E-mail: kenta.amemiya@kek.jp
Professor Hiroshi Kondoh
Department of Chemistry Faculty of Science and Technology,
Keio University, Japan
Tel: 81-45-566-1701
E-mail: kondoh@chem.keio.ac.jp
[for public relations]
Youhei Morita
Public Relations Office,
High Energy Accelerator Research Organization, Japan
Tel: 81-29-879-6047
Fax: 81-29-879-6049
E-mail: press@kek.jp
Office of Communications and Public Relations, Keio University, Japan
Tel: 81-3-5427-1541
E-mail: m-koho@adst.keio.ac.jp
http://www.keio.ac.jp/index-en.html
Glossary
*1 Monolayer
The quantity of molecules that cover the surface of a solid with a single layer.
*2 Heterogeneous catalyst
Unlike a homogenous catalyst, which is in the same fluid phase as the reagents and has a singular and defined structure, a heterogeneous catalyst is in a different phase from the reagents (most often the solid state) and refers to a catalyst that sees gases or liquids react at its surface.
*3 Spectroscopy
A technique in which the wavelength (energy) dependence of the absorption or reflection of irradiated light (eg. infra-red, ultra-violet, or X-ray regions) by a target sample is measured.
*4 Undulator
An undulator is a device that consists of magnets in which a south pole and a north pole are alternately placed in a row, and two rows of these magnets are aligned in parallel so that a south pole in one row faces a north pole in the opposite row. When electron beams are injected into an undulator, electrons wiggle to emit synchrotron radiation light while passing through it by receiving an alternate direction of the magnetic force. By utilizing undulators, higher intensity and more directional X-rays can be produced.
*5 Polarization switching
Light (including soft X-ray) has a wave nature. When a vibration plane of a light wave is in a horizontal plane or in a vertical plane, the light is called horizontally polarized or vertically polarized, respectively. Comparison of the soft X-ray absorption spectra using horizontally polarized X-rays with those using vertically polarized X-rays can identify the directions (orientations) of molecules on the sample surface. Polarization switching is a method in which the polarization direction of light is alternately switched between horizontal and vertical directions at high frequency (many times per second), enabling the continuous measurement of the temporal changes in the orientations of molecules over the course of the reaction of interest.