Press Release
Observation of How an Organic Molecule Mimics Photosynthesis
March 6, 2012
Tokyo Institute of Technology, Japan
High Energy Accelerator Research Organization (KEK), Japan
Osaka University, Japan
Japan Science and Technology Agency
Extensive research is being carried out on artificial photosynthesis, a technique for mimicking the photosynthesis mechanism of plants at the molecular level, for the efficient utilization of solar energy, which is gaining popularity as a sustainable next-generation energy source.
The research groups at Tokyo Institute of Technology, KEK, and Osaka University carried out direct molecular and crystal structure observations to elucidate the structural mechanism by which 9-mesityl-10-methylacridinium ions, which are photofunctional species mimicking the solar energy conversion process in photosynthesis, efficiently convert the absorbed optical energy into chemical energy, which is then preserved for a duration longer than 1000 times that in natural photosynthesis.
The research groups used Photon Factory (PF-AR) to carry out pump-probe X-ray diffraction and single-crystal X-ray structural analysis, with the aim of investigating the molecular structure immediately after the conversion of optical energy into chemical energy by the 9-mesityl-10-methylacridinium ions synthesized by the research team at Osaka University. 9-Mesityl-10-methylacridinium ions underwent structural changes upon the absorption of light. The methyl group was bent away from the mean plane of the acridine site, and the perchlorate ion that coexisted in the crystal moved closer to the mesitylene site (Figure 2). The results were obtained from the first direct observation, where the efficient energy conversion in 9-mesityl-10-methylacridinium ions was found to be related to the photoinduced electron transfer from the mesitylene site to the acridine site, as was expected when designing the molecule.
An important observation was that the mesitylene and acridine sites in 9-mesityl-10-methylacridinium ions remained orthogonally aligned throughout the energy conversion process. The orthogonal alignment strongly restricted the electron from being transferred to the original position, supporting the result that the converted energy is retained for a long duration.
Pump-probe X-ray diffraction, which is a very new measurement method, is used for the first time to investigate an artificial photosynthesis system and obtain significant results. These findings will provide a powerful strategy for the development of new artificial photosynthesis systems.
Publication |
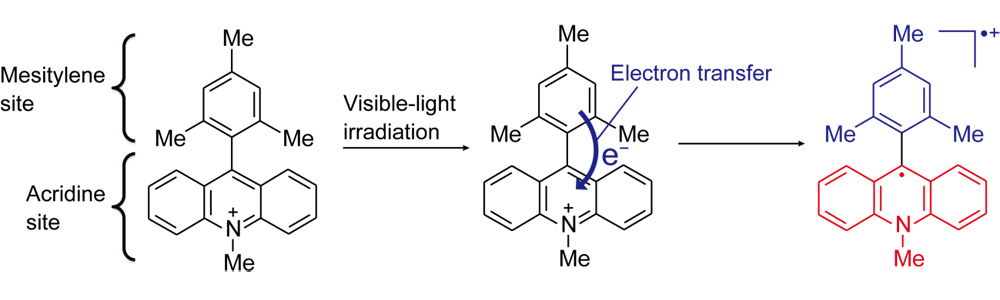
Figure 1: The 9-mesityl-10-methylacridinium ion.
An electron is transferred from the mesitylene site (blue) to the acridine site (red) after the absorption of visible light, and thus, the absorbed optical energy is converted into chemical energy.
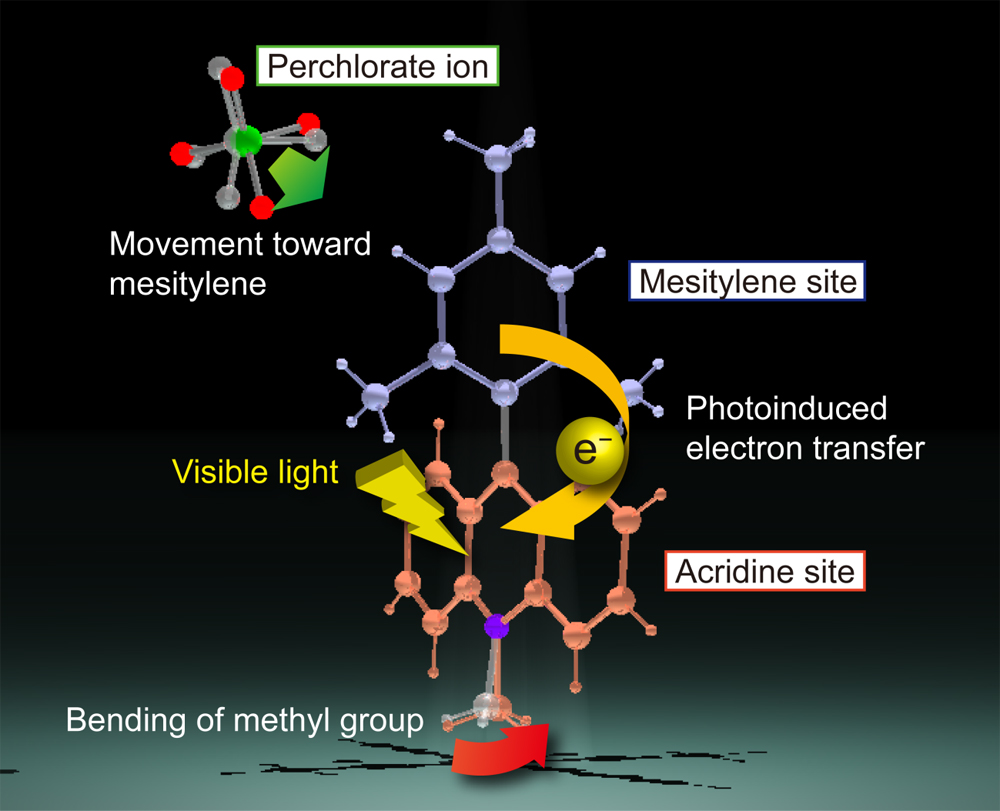
Figure 2: Structural change accompanying the observed energy conversion
When light is absorbed and converted into chemical energy, the methyl group bonded to the acridine site bends and the adjacent perchlorate ion in the crystal moves closer to the mesitylene site. The relative positions of the mesitylene and acridine sites do not change within the resolution of observation.
Media Contact
[for research]
Manabu Hoshino
Tokyo Institute of Technology, Japan
Tel: 81-29-879-6185
Fax: 81-29-879-6187
E-mail: mhoshino@chem.titech.ac.jp
[for public relations]
Youhei Morita
Public Relations Office, High Energy Accelerator Research Organization, Japan
Tel: 81-29-879-6047