A pair of ions behaves as a surfactant molecule
-Novel hierarchic structure observed in a liquid mixture - From KEK
 |
A hierarchical structure with multilamellar structure in nano-meter scale and a spherical onion structure in micro-meter scale are observed in an off-critical mixture of D2O and 3-methylpyridine (3MP) containing sodium tetraphenylborate. Prof. Seto and Mr. Sadakane at KEK, Profs. Onuki and Nishida at Kyoto University and Dr. Koizumi at Japan Atomic Energy Agency have confirmed the novel structures in a liquid mixture without surfactant by small-angle neutron scattering (SANS) and by optical microscopy measurements.
Figure 1 shows the temperature dependence in the optical microscopic images. At T = 50 ºC, the entire area is homogeneous without visible structures. With decreasing temperature below TL=45 ºC, small droplets emerged from the entire sample in Fig. 1(b) and they grew in size. In Fig. 1(c), T is further lowered to 20 ºC and the space is nearly filled with deformed droplets having diameters of approximately 20 μm. The left-hand side panel of Fig. 1(b') shows a magnified view of Fig. 1(b), while the right-hand side panel shows the corresponding Maltese cross pattern obtained under crossed Nicoles using polarized light. This pattern arises from inhomogeneous composition distribution with spherical symmetry like onions.
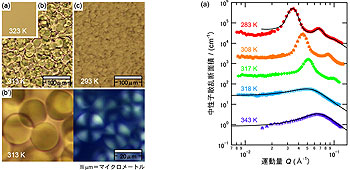
Figure 2 shows the SANS intensity vs wave number Q and its temperature dependence. It was found that the system was in the disordered phase for T > TL=45 ºC. The multi-peak profile below TL indicates the formation of the ordered structure. This profile is well explained by the formula for lyotropic lamellae as shown by the solid curve of T = 10 ºC in Fig. 2 (a). The spatial distribution of the scattering density profile is reconstructed as shown in Fig. 2 (b). The experimental result can be interpreted by the existence of flat membranes of 3MP with hydrophobic anions are formed, and hydrophilic cations distribute near the membranes.
The salt consists of hydrophilic cations and hydrophobic anions that interact asymmetrically. The cations and anions are surrounded by water molecules and by 3MP molecules, respectively. These clusters are attracted each other by the electrostatic force and decrease the surface tension. This effect is analogous to that of surfactant molecules. This could be the origin of the emergence of the layered structure in the mixture with water, 3MP and the salt in the absence of a surfactant.
This result was appeared in Physical Review Letters published in October 16, 2009.
Figure 1 : Optical microscopic images oftained from the mixture of D2O, 3MP and sodium tetraphenylborate; (a) T = 50 ºC (disordered), (b) T = 40 ºC (onion), and (c) T=20 ºC (onion). Scale bars in (b) and (c) are 100μm. (b') A magnified view of (b) (left) and a birefringent image of the same region (right) with the scale bar being 20 μm.
Figure 2 : (a) Temperature dependence of SANS intensity. (b) Scattering density distribution at T = 10 ºC estimated from the theoretical curve (left) and schematic picture of the distribution of the ingredients (right). The vertical axis indicates the distance normal to the layers.
