Structural biology of protein modification and transport processes
Protein modification and sorting are essential for all living cells. In higher organisms such as human, newly synthesized proteins need to be modified and directed to their correct targets for proper functioning. Error in protein modification and mis-targeting of proteins into wrong compartments cause a number of human diseases. Furthermore, infection and proliferation of viruses such as HIV and influenza require host cell’s transport systems. Hence, elucidation of detailed mechanisms of protein modification and transport is expected to lead to development of new drugs against diseases caused by viruses and mis-targeted proteins. In particular, two compartments called the endoplasmic reticulum and the Golgi apparatus are the key to protein modification and correct sorting. In analogy to chemical plants, these compartments serve both as a distillation tower and as a chemical reactor of the cell by sorting and modifying proteins. In the long run, full understanding of these mechanisms will be extremely useful in developing key technologies to produce medically/ biologically active human glycoproteins (proteins modified with short sugar chains) using lower organisms such as yeast.
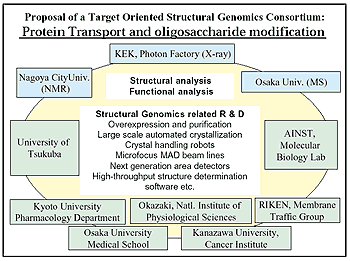
To pursue these lines of research, the PF structural biology group, together with five universities and three institutes, is proposing a SG project on protein transport and post-translational modification (which means modification after proteins are produced from the genes) of proteins as a consortium. The core of this consortium has already began in the form of the collaboration between the PF group and the K. Nakayama’s group in University of Tsukuba and Y. Jigami’s group in AINST, Tsukuba. The project includes systematic structural analyses of proteins and complexes involved in protein transport and post-translational modification processes, using synchrotron X-ray protein crystallography, NMR spectroscopy, and mass spectroscopy. The proposal will give high priority to development of various high-throughput protein crystallography techniques using synchrotron radiation.
