 |

|
|
|
The new Structural Biology Group
|

The new PF Structural Biology Group started in May 2000 with the emphasis on combining synchrotron beam line activities and competitive structural biology research. The group has currently 15 members (1 professor, 1 associate professor, 5 research associates, 1 computer scientist, 2 post doctoral fellows, 1 software engineer, 1 technician, 1 mechanical engineer from the KEK Mechanical Engineering Center, and 2 secretaries) covering a wide spectrum of the research fields ranging from the beam line development and operation, software development, biochemistry, and protein crystallography. At least four post-docs and two Ph.D. students will join the group from April 2002. We expect to open additional positions shortly and invite applications from abroad.
|

A new Structural Biology Building was completed in March 2001. The onestory 438 m2 building houses two main biology laboratories for biochemistry and genetic engineering with ancillary laboratories for protein expression, purification and crystallization. It is also possible to carry out simple characterization of proteins and their interaction, for instance surface plasmon resonance experiment. The computer graphics room has several workstations for solving and analyzing protein structures.
|


Since the completion of the building, the group has initiated a number of structural biology research projects focused on the intracellular protein transport and post-translational protein glycosylation. One of them is a structural study of a small domain called VHS of a human GGA1 protein involved in protein transport in cells. The human GGA protein has been the focus of intense research of many cell biologists in the US, Europe and Japan during the last 12 months. Several groups including Prof. Kazuhisa Nakayama of Tsukuba University with whom our group has a close collaboration, simultaneously found the protein. There have been a number of important papers reported in the literature on the biological role of the protein during the past 12 months, notably one in Cell and two in Science. In short, the protein is involved in the interaction with sorting receptors and clathrin molecules, and therefore is believed to play a key role in the formation of transport vesicles.
|


Protein preparation was started on 23 April 2001, the same month as the completion of the building, and the structure was solved five weeks later on 28 May 2001. In order to understand the mechanism of the signal peptide recognition, we subsequently tried to solve the structure of the domain in complex with the receptor fragment. By the time we finally obtained crystals of the complex, it was already August and many synchrotron facilities were shut down for summer recess. However, Dr. Thomas Earnest of the ALS, Berkeley, U.S.A., has offered to collaborate. So we sent frozen crystals to Berkeley at 5PM 13th August last year by Federal Express. As soon as he received the crystals, he collected diffraction data sets and 44 hours after the shipment from Tsukuba, I received the data by E-mail. We were all extremely pleased when a quick calculation confirmed the binding of the signal peptide! The result was published in Nature vol. 415, 937-941, 21 February 2002. A group in the National Institute of Health in the USA also solved the structure of the complex from GGA3, 73 percent identical to GGA1.
|
Their paper appeared in the same issue of Nature. In the future, the structures of the other domains will help elucidate the molecular mechanism of the protein-protein interaction of this protein which is critical for correct transport of proteins from the terminal Golgi network to lysosomes (a compartment which degrades unwanted macromolecules) using sorting receptors. The group is extending this study to other related proteins and their complexes with the hope to gain atomic details of protein transport and modification processes.
Further information is available from Prof. Soichi Wakatsuki, IMSS, KEK, 1-1 Oho, Tsukuba, Ibaraki 305-0801, Japan, Soichi.Wakatsuki@kek.jp, Tel: +81-298-64-5648, Fax: +81-298-64-2801. Web site is under construction: http://pfweis.kek.jp

|
 |
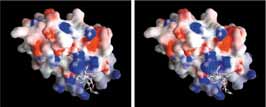
Electrostatic surface potential (red: negative, blue: postitive) of the GGA1-VHS domain in complex with the sorting receptor fragment shown as a stick model. Above pictures are for a stereo-vision. Use your left eye for the left picture, right eye for the right picture and try to merge them together. This will give you a 3-dimentional view of the protein. |
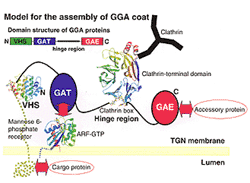
GGA proteins are composed of a VHS domain, a GAT domain, and a GAE domain. The GAT domain is linked to the GAE domain by a flexible hinge region. The VHS domain directly interacts with the acidic cluster dileucine motif in the cytoplasmic domain of sorting receptors. The GAT domain binds to the GTP-bound form of ARFs. The hinge region binds clathrin. The GAE domain binds to various accessory proteins.
|
|
The author of this article, Soichi Wakatsuki, obtained a Ph.D. in Chemistry from Stanford University, worked as a PDF at the University of Oxford, UK. He then moved to the European Synchrotron Radiation Facility, France and lead the Structural Biology Group. After 16 years working abroad, he has made a full circle, at least for the time being, and assumed a position as the head of the new Structural Biology Group at the Photon Factory, IMSS, KEK in May 2000.
|
|
|

|

copyright(c) 2008, HIGH ENERGY ACCELERATOR RESEARCH ORGANIZATION, KEK
1-1 Oho, Tsukuba, Ibaraki 305-0801 Japan |
mailto : ohska post.kek.jp post.kek.jp |

|
|
