Getting through the bottleneck--A new class of layered perovskite with high oxygen-ion conductivity
#IMSS #Pressrelease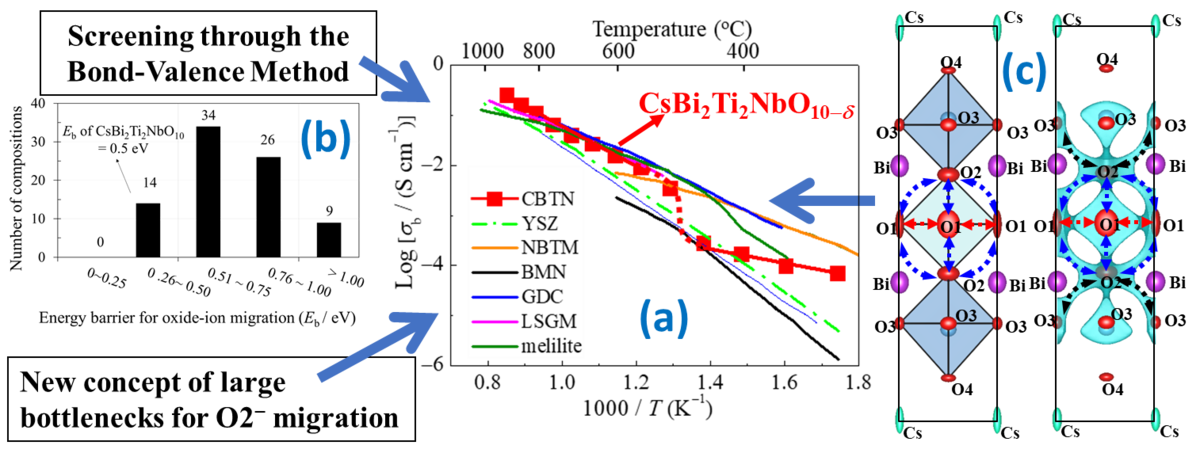
- Tokyo Institute of Technology (Tokyo Tech)
- High Energy Accelerator Research Organization (KEK)
- Japan Proton Accelerator Research Complex (J-PARC)
Scientists at the Tokyo Institute of Technology (Tokyo Tech) have discovered a layered perovskite that shows unusually high oxide-ion conductivity, based on a new screening method and a new design concept. Such materials are hard to come by, so the discovery of this material, the new method and design concept will make the realization of many environment-friendly technologies.
Upon hearing the word “conductor” in reference to chemistry, most will immediately think of the movement of electrons within a material. But electrons are not the only particles that can move across a material; oxide ions can too. Many materials scientists and engineers are currently searching for materials with high oxide-ion conductivity. Such materials have many potential applications, particularly in the development of environmentally friendly technologies. For example, oxide-ion conductors could be used in fuel cells, which directly convert clean fuel such as hydrogen into electrical energy, or in oxygen separation membranes, which could be useful in systems for capturing the CO2 we produce by burning coal or fossil fuels.
Unfortunately, high oxide-ion conductivities can be achieved by a limited number of structure families of materials. The perovskites are one such structure family. Perovskites and layered perovskites have special crystal structures that sometimes exhibit outstanding physical and chemical properties. Prof. Masatomo Yashima and colleagues from the Tokyo Institute of Technology studied a class of layered perovskites, a Dion-Jacobson phase, where two-dimensional perovskite-like “slabs” are stacked and separated by a layer of alkali metal ions, such as the cesium cation (Cs+). In their paper published in Nature Communications, Professor Yashima and his colleagues explain their motivation:“Numerous studies have been conducted on the electrical properties of Dion-Jacobson phases, such as their proton, lithium-ion and sodium-ion conduction. However, there are no reports on the oxide-ion conduction in Dion-Jacobson phases.”
Please refer to the press release for details.