◆ 論文等
◆ 論文等
◆ 学位論文
◆ 研究成果
High Proton Conductivity in Nb5+-Doped BaScO2.5: Advancing Proton-Conducting Materials for Clean Energy
Kei Saito, Mitsuki Baba, Kensei Umeda, Kotaro Fujii, Takashi Honda, and Masatomo Yashima
This groundbreaking research presents a significant advancement in the development of high-performance proton-conducting ceramics, focusing on a novel material: Nb5+-doped BaScO2.5. These materials are promising candidates for electrolytes in clean energy devices such as protonic ceramic fuel cells (PCFCs), which aim to operate efficiently at lower temperatures, reducing costs and enhancing durability. The study demonstrates that by doping BaScO2.5 with pentavalent niobium (Nb5+), it is possible to achieve extraordinary proton conductivity levels—around 0.01 S/cm at 320°C—among the highest reported for ceramic proton conductors below 200°C. This remarkable performance results from a combination of high proton concentration, diffusion coefficient, and chemical stability. This high conductivity is attributed to two main factors: a large number of oxygen vacancies and full hydration in the material [1], producing a large number of mobile protons. The low activation energy for proton diffusion (~0.44 eV) further facilitates rapid proton transport. Ab initio molecular dynamics simulations reveal the electrostatic repulsion between Nb⁵⁺ dopant and protons, reducing the proton trapping compared with the conventional acceptor-doped perovskites, allowing the higher mobility at lower temperatures. Additionally, the structure remains thermally and chemically stable even under humid conditions, which is critical for practical applications.
A new aspect of this study is the doping of pentavalent donor Nb5+. The Nb5+ doping not only stabilizes the cubic phase of BaScO2.5 but also optimizes the number of oxygen vacancies for water incorporation and proton conduction. The structural analysis using neutron diffraction data confirms the successful water incorporation as OH- anion (Fig. 1a). The bond-valence-based energy landscape suggests the proton diffusion pathways (Fig. 1b).
This approach offers a promising pathway to design the next-generation proton conductors by intentionally introducing intrinsic oxygen vacancies and employing donor doping to mitigate proton trapping. The improved conductivity at low and intermediate temperatures significantly enhances the prospects for solid oxide fuel cell applications, offering higher efficiency and durability. Overall, this study highlights that manipulating the defect chemistry and doping strategies in oxides can lead to materials that will revolutionize energy conversion technologies, especially in the clean and sustainable energy sector. This work was published in Inorganic Chemistry Frontiers [2]
References
[1] K. Saito et al., J. Mater. Chem. A, 12, 13310–13319 (2024).
[2] K. Saito et al., Inorg. Chem. Front., 2025, Advance online publication. DOI: 10.1039/D5QI00632E
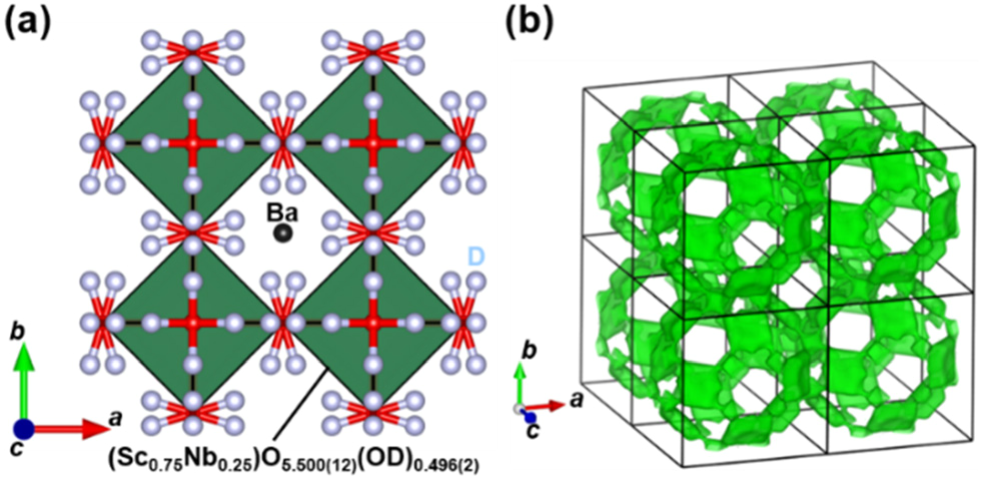
Fig. 1 (a) Refined crystal structure of BaSc0.75Nb0.25O3D0.5 (BSN25) at −243 °C, which was represented using (Sc0.75Nb0.25)O5.500(12)(OD)0.496(2) octahedra. Ba, O, and D atoms are denoted by the black, red, and grey spheres, respectively. The red/grey lines denote the OD bonds. (b) Isosurface of the bond-valence-based energy at 0.45 eV for a test proton of BSN25, which was calculated for the crystal parameters refined using the neutron diffraction data of BSN25 at −243 °C. Energy barrier for proton migration was estimated to be 0.38 eV, which was consistent with the experimental activation energy for bulk diffusion coefficient 0.44 eV. Black square denotes a unit cell [2].
◆ 論文等
◆ 論文等