◆ 論文等
◆ 学位論文
◆ 受賞
◆ 研究紹介
Column-like La Arrangement Unveiled in High-Performance Lithium-Ion Conductor: A Breakthrough in Solid-State Electrolyte Design
Koji Kimura, Shunya Yamazaki, Naoto Kitamura, Yasuhiro Takabayashi, Hiroaki Takeda, Ryo Kobayashi, Hiroo Tajiri, Takashi Honda, and Koichi Hayashi
Researchers have successfully applied X-ray Fluorescence Holography (XFH) to a single crystal of the lithium-ion conductor (Li0.15La0.85/3)NbO3 (LLNO), providing local structural details about the arrangement of La3+ ions. This discovery challenges the long-held structural model for LLNO and suggests a direct connection between the unique La3+ arrangement and the material's enhanced ionic conductivity. The conventional model for LLNO, a well-known solid-state lithium-ion conductor with a perovskite structure, posits that La3+ and Li+ ions reside in alternating La-/Li-rich (A1) and La-/Li-empty (A2) layers along the c axis. However, the XFH experiments directly revealed that a fraction of La3+ ions actually occupies the A sites within the supposedly empty A2 layers. This observation was only possible due to the advantage of XFH, which can detect atomic correlations at distances greater than 5.5 Å, well beyond the reach of techniques like EXAFS.
A detailed analysis of the reconstructed XFH atomic images, comparing experimental results to various structural simulations (random, one-column, and four-column models), indicated that the La3+ ions are not randomly distributed. Instead, the La3+ ions aggregate to form column-like arrangements with an approximate size of ∼15×15 Å2 in the ab plane. In this structure, La3+ ions in the A2 layers (La2 ions) are aligned in the c-axis direction, and those in the A1 layers are aggregated near these La2 ions. The calculated atomic image intensity ratio for the four-column model showed the best agreement with the experimental data, strongly supporting this aggregation model.
The existence of La3+ ions in the A2 layers is crucial for performance. The LLNO single crystal used in this study, which was grown by the Czochralski method, showed a high ionic conductivity of σ[100] = 2.8×10−4 S⋅cm−1 at 306 K. This value is higher than those reported for similarly composed LLNO samples grown by the Bridgeman method. This enhanced conductivity is attributed to the presence of La3+ ions in the A2 layers. The transfer of La3+ ions from A1 to A2 layers allows Li+ ions to migrate not only within the A1 layers but also to enter the A2 layers, which dramatically expands the migration space and enhances Li+ ion conduction. The column-like arrangement is believed to be caused by the larger temperature gradient during the crystal growth process, which can more easily maintain the metastable states that favor La3+ occupancy in the A2 layers. This research highlights that controlling the La3+ arrangement is a key to maximizing Li+ ion conductivity in solid-state electrolytes.
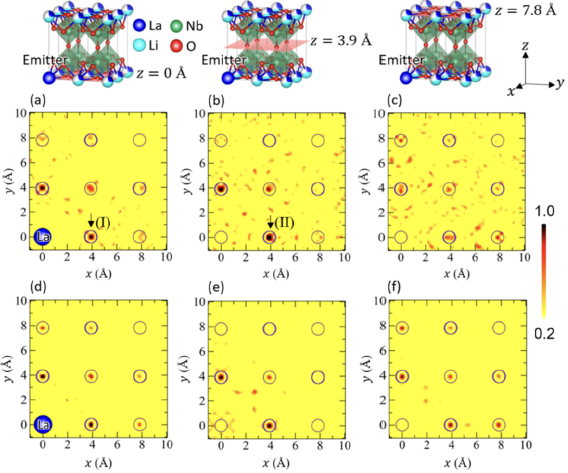
Fig. 1. Atomic images reconstructed from XFH experiments (a-c), and those calculated using the four-column model (d-f). The planes are z = 0 Å, z = 3.9 Å, and z = 7.8 Å. The clear atomic images in the middle panel (z = 3.9 Å, the A2 layer) confirm La3+ occupation in the conventionally "empty" layer.
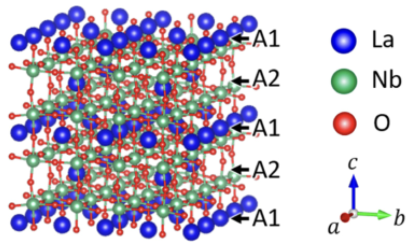
Fig. 2. Structural model of the local La3+ ionic arrangement in LLNO: the four-column model.
◆ 論文等